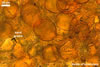
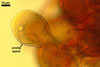
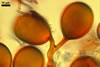
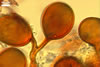
GERMINATION.
Not observed.
MYCORRHIZAE.
Glomus aureum has formed vesicular-arbuscular mycorrhizae in one-species cultures with Plantago lanceolata L., Fragaria x ananassa Duchesne, Allium porrum L., Glycine max (L.) Merr., and Triticum aestivum L. as the host plants (Oehl et al. 2003).
DISTRIBUTION. The holotype of Gl. aureum has been chosen from spores isolated from a pot culture inoculated with a spore cluster recovered from a grassland located near Therwill, Switzerland (Oehl et al. 2003). Additionally, this fungus has been found among roots of plants of other grasslands located near Vogtsburg in Kaiserstuhl (Germany), Leymen, Department Haut Rhin (France), Assisi, Umbria (Italy; Oehl et al. 2003, 2005), in agricultural soils at Thervil (Oehl et al. 2004), as well as under a vineyard and in two mono-cropped maize fields placed between Basel (Switzerland), Freiburg i. Br. (Germany) and Mulhouse (France; Oehl et al. 2005).
NOTES.
The morphological and biochemical properties of spores and mycorrhizae of Gl. aureum presented above were prepared based on the original description of this fungus (Oehl et al. 2003) and observations of its spores loaned from Dr. F. Oehl, Institute of Botany, University of Basel, Switzerland.
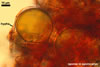
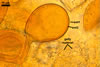
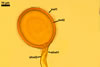
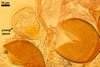
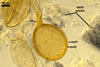
According to Oehl et al. (2003), the structure appearing at the beginning of the development of Gl. aureum is a cluster of hyaline mycelium, inside which gradually spores origin. The spores are first hyaline and later gradually darken due to the synthesis of next sublayers of the second laminate spore wall layer.
Because the amorphous gel filling the spaces between hyphae and spores stains identically to the outer layer of the spore wall, the subtending hyphal wall and the hyphal wall of the sporocarpic interior, Oehl et al. (2003) concluded it to be a conglomerate of sloughed fragments of this layer.
Of Glomus spp. forming coloured sporocarps, three groups include species more or less similar to Gl. aureum. The species morphologically closest of Gl. aureum are Gl. glomerulatum Sieverd., Gl. pallidum I.R. Hall, Gl. proliferum Dalpe & Declerck, and Gl. rubiforme (Gerd. & Trappe) R.T. Almeida & N.C. Schenck. Spores of all these fungi are yellow-coloured and occur in compact sporocarps without a peridium (Błaszkowski 2003; Declerck et al. 2000; Gerdemann and Trappe 1974; Hall 1987; Oehl et al. 2003; Sieverding 1987).
The main properties readily separating Gl. glomerulatum from Gl. aureum are darker spores (up to brown) of the former species, their inner flexible hyaline layer (no such a layer in spores of the latter fungus), and the intercalary mode of origin of the spores, whose the result is that all spores have two subtending hyphae (Sieverding 1987; vs. spores with only one subtending hypha in Gl. aureum).
Compared with Gl. pallidum, spores of Gl. aureum may be darker [light orange (5A4) to orange (5A7; Błaszkowski, pers. observ.) vs. cream (4A3) to pale orange (5A3)], their structural subtending hyphal wall is of the same colour as the structural spore wall layer (vs. it is hyaline up to the spore base), and the outer spore wall layer reacts in Melzer's reagent (Błaszkowski, pers. observ.; Oehl et al. 2003; vs. no reaction).
Glomus proliferum differs from Gl. aureum in the more complex structure of the spore wall. While the spore wall of the latter species comprises four layers (Declerck et al. 2000), only two layers form the spore wall of the former fungus.
Glomus rubiforme distinguishes the side by side arrangement of its spores in a single hemispherical layer around a hyphal plexus (Błaszkowski 2003; Gerdemann and Trappe 1974). In G. aureum, its spores are randomly distributed in sporocarps.
The second group of fungi mentioned above represents Gl. invermaium I.R. Hall. In contrast to Gl. aureum producing compact sporocarps with spores, whose the outer wall layer deteriorates with age, spores of Gl. invermaium occur in loose sporocarps and their outer wall layer is persistent (Błaszkowski, pers. observ.; Hall 1977).
The third group includes Gl. microcarpum Tul. & C. Tul. and Gl. vesiculiferum (Thaxt.) Gerd. & Trappe that diverge from Gl. aureum in the formation of sporocarps with a peridium more or less covering their spores (Berch and Fortin 1984; Gerdemann and Trappe 1974; vs. no peridium in Gl. aureum). Moreover, the peridium of Gl. vesiculiferum uniquely distinguishes this species, because it is composed of thin-walled, pear-shaped or broadly clavate, whitish vesicles (Gerdemann and Trappe 1974).
REFERENCES
Berch S. M., Fortin J. A. 1984. A lectotype for Glomus microcarpum (Endogonaceae, Zygomycetes). Mycologia 76, 190-193.
Błaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone, and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland. http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Declerck S., Cranenbrouck S., Dalpé Y., Séguin S., Grandmougin-Ferjani A., Fontaine J., Sancholle M. 2000. Glomus proliferum sp. nov.: a description based on morphological, biochemical, molecular and monoxenic cultivation data. Mycologia 92, 1178-1187.
Gerdemann J. W., Trappe J. M. 1974. The Endogonaceae in the Pacific Northwest. Myc. Memoir 5, 1-76.
Hall I. R. 1977. Species and mycorrhizal infections of New Zealand Endogonaceae. Trans. Brit. Mycol. Soc. 68, 341-356.
Oehl F., Wiemken A., Sieverding E. 2003. Glomus aureum, a new sporocarpic arbuscular mycorrhizal fungal species from European grasslands. J. Appl. Bot. 77, 111-115.
Oehl F., Sieverding E., Mader P., Dubois D., Ineichen K., Boller T., Wiemken A. 2004. Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia 138, 574-583.
Oehl F., Sieverding E., Ineichen K., Ris E.-A., Boller T., Wiemken A. 2005. Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytol. 165, 273-283.
Sieverding E. 1987. A VA-mycorrhizal fungus, Glomus glomerulatum sp. nov., with two hyphal attachments and spores formed only in sporocarps. Mycotaxon 29, 73-79.